
Treatment of Severe Atopic Eczema Trial (project complete)
A Randomised Controlled Trial Assessing the Effectiveness, Safety and Cost-effectiveness of Methotrexate versus Ciclosporin in the Treatment of Severe Atopic Eczema in Children: The TREatment of Severe Atopic Eczema Trial (TREAT)
About the project
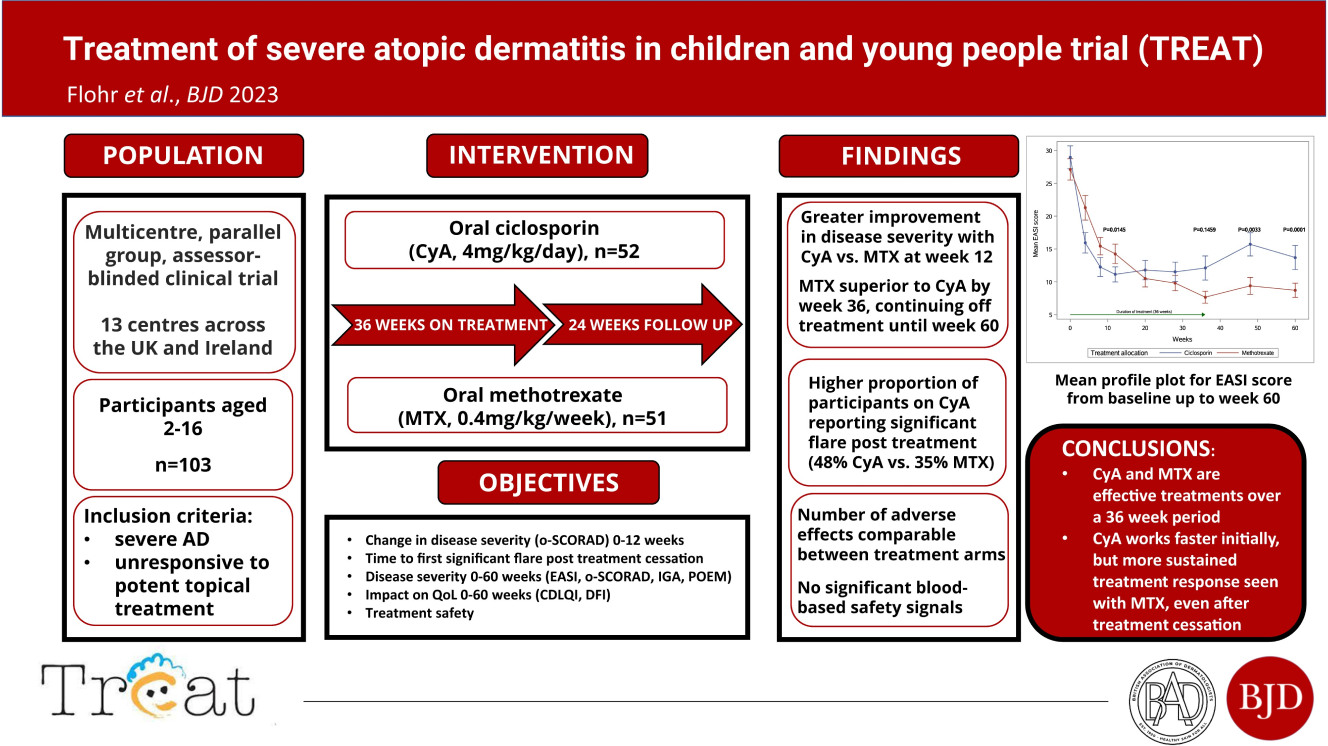
The TREAT (TREatment of Severe Atopic Eczema Trial) study was a national multi-centre randomized controlled trial that assessed the effectiveness, safety, and cost-effectiveness of methotrexate versus ciclosporin in treating severe atopic eczema in children.
Led by Professor Carsten Flohr, Consultant Paediatric Dermatologist at St John’s Institute of Dermatology, Guy’s & St Thomas’ NHS Foundation Trust, the trial aimed to recruit 102 patients aged 2-16 years requiring systemic treatment. Participants were randomly allocated to receive either methotrexate or ciclosporin for 9 months, followed by a 6-month follow-up period to evaluate short- and long-term effectiveness as well as the safety profile of both drugs. TREAT also addressed gaps in current knowledge regarding how these medicines reduce skin inflammation and itch.
Recruitment has now closed
The recruitment process is now complete. You can read through this site to understand the study and its results.
Study overview video
Find out about the trial from Prof Carsten Flohr. Understand the reason for the trial, how it was conducted and the results.
Summer and Stephanie’s experience
Find out about Summer and her mum Stephanie’s experience with the TREAT trial. With interviews from Prof Carsten Flohr and Charlotte Walker.

Hospital departments taking part
The study will take part in Dermatology Departments across the country. To see if your hospital is taking part in this study please go to recruiting centres.

Study funder
The study will take part in Dermatology Departments across the country. To see if your hospital is taking part in this study please go to recruiting centres.

Study organiser
The study will take part in Dermatology Departments across the country. To see if your hospital is taking part in this study please go to recruiting centres.

Study review
The study has been reviewed by a research ethics committee, who have agreed the study is being conducted in a correct and appropriate manner. The study has also been approved by the Medicines & Healthcare products Regulatory Agency (MHRA).
The British Journal of Dermatology
Useful links
Trial summary and frequently asked questions
We are doing this study (called TREAT) to find out which is the best medicine to give children and young people who have severe eczema.
The two medicines that are being tested in this study are called Methotrexate and Ciclosporin. These medicines are not new and are already used in the treatment of children and young people who have severe eczema. We would like to find out which is the best to use.
You have been diagnosed with severe eczema which is difficult to manage and your hospital is taking part in the study.
We would like to involve 102 children and young people aged between 2 and 16 years. The information from this study will help us to improve how we treat children and young people who have severe eczema.
No not at all. It’s completely up to you. We only want you to take part if you want to. Just tell us if you don’t.
If you decide not to, don’t worry, it won’t change how you are looked after.
If you decide to take part, you will be given this leaflet to keep. You will be asked if you would like to sign a form to say that you understand what will happen and that you are happy to take part. Your parent/carer(s) will also have to sign a form to say they are happy for you to take part.
If you decide to take part that would be really helpful. If you then change your mind, that’s OK as well – you can, and don’t have to say why if you don’t want to.
You will be in the study for a total of 15 months. You will be asked to take the study treatment for the first 9 months. You will have one more visit to your hospital clinic than you would usually have.
You will be told at your second visit whether you are in the group taking Methotrexate or Ciclosporin. To make it fair, no-one will pick which of the two medicines you are going to be given. It will be decided by a computer. You have the same chance of getting either medicine.
You may need to have an X-ray on your chest before taking any study medicine to check you are healthy.
The following will happen at most visits:
- Your eczema will be assessed
- You will be asked to fill in some short forms about how you are getting on
- Your blood pressure will be taken
- Your height and weight will be measured
- You will be given some more study medicine
If you are in the Methotrexate group, you will be asked to take the medicine (either as a tablet or an injection) once a week. If you are in the Ciclosporin group you will be asked to take the medicine (as a tablet) twice a day. If you are in the Methotrexate group you will be asked to come to the hospital for one more visit than the Ciclosporin group.
During the time you are receiving the study medicine you will be asked to give a blood sample at your clinic visits. You can have a cream or spray to numb the skin first if you would like. The blood samples will be used to check how you are getting on with taking the medicine. You will also be asked to give a urine sample every 3 months during the time you are receiving the study medicine. At the last visit, you will also be asked to give a blood sample and a urine sample. We will also ask if you can give a saliva sample at one visit.
At some of the visits, we will also use a sticky tape to collect cells from the surface of your skin. This does not hurt. It’s just like putting sticky tape on your skin and gently lifting it up.
You will be asked to fill in a diary at home to keep a record of how you are getting on with the study medicine and your eczema. You will also need to record when you have taken your study medicine.
You will still be able to use your eczema creams whilst you are taking part in the study.
There are no more risks in taking part in the study compared to not taking part. These medicines are already used in normal care, but like other medicines sometimes they have unwanted symptoms (side effects). The research team will keep a close eye on you by doing some tests to check how you are getting on with taking the medicines and to check if there have been any side effects.
If you are in the Methotrexate group, you could get some of the following side effects:
- Feeling sickly
- Feeling tired
- Headache
- Indigestion (upset stomach)
- Diarrhoea (loose poo)
- Sore mouth or sore throat
- Loss of appetite (don’t feel like eating)
If you are in the Methotrexate group, you should not take Ibuprofen as the two medicines can cause harm if taken together.
If you are in the Ciclosporin group, you could get some of the following side effects:
- Headache
- Shakiness
- Feeling sickly, diarrhoea (loose poo), tummy pain
- Tiredness
- Fever
- Acne
- Muscle cramps and muscle pain
- Pins and needles
- Hair growth on the face or body
- Sore gums
When you start taking the medicines it can sometimes lower the number of blood cells found in your blood which help you fight off infection and help stop bleeding and bruising, so you could be more likely to get an infection. The medicines could also affect your liver and kidneys but the research team will monitor any changes in your blood, liver and kidneys regularly. If you do get any side effects they usually go back to normal when you stop using the medicines.
If you notice any side effects it is important to tell your parent or the grown up who looks after you straight away so that they can tell your research doctor.
It is possible that if the study treatment is given to a pregnant female it could harm an unborn baby. You must not take part in the study if you are pregnant; neither should you if you plan to become pregnant during the study. We will test your urine to see if you are pregnant. Both males and females (who are sexually active) taking part in the study must agree to use contraception (a way of preventing pregnancy) whilst they are receiving the study medicine and for 6 months after the study medicine has been stopped. Any female who finds that she is pregnant while taking part in the study should tell her research doctor straight away. Any male whose partner becomes pregnant while taking part in the study should also tell his research doctor straight away.
We cannot promise the study will help you, but both medicines have been shown to improve eczema in most cases. We hope that the information we get from this study will improve the lives of other children and young people through better treatment for their eczema in the future.
The study is being run in your hospital, and is being organised by King’s College London and Guy’s and St Thomas’ NHS Foundation Trust and the University of Liverpool.
Your mum, dad or the grown up who looks after you have been given lots of information.
Funded by National Institute for Health Research's Efficacy and Mechanism Evaluation (EME) Programme

Contact us
Get in touch to find out more about the TREAT trial
- Address
Medicines for Children Clinical Trials Unit Clinical Trials Research Centre University of Liverpool Institute of Child Health Alder Hey Children’s NHS Foundation Trust Liverpool, L12 2AP










